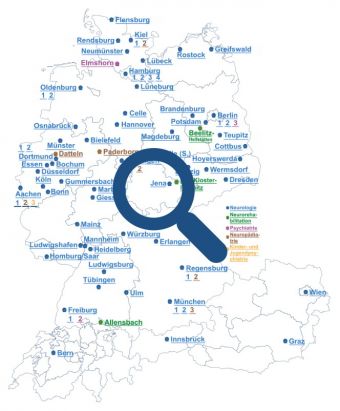
The GENERATE Network structure
The GErman NEtwork for Research on AuToimmune Encephalitis is comprised of individual inter-disciplinary groups of experts working in basic and clinical research, linked by a central national patient register. The network currently consists of more than 100 centres.
The GENERATE network is complemented by the registered non-profit association GENERATE e.V., which was founded in Vienna in January 2017. With the establishment of a registered association, the existing NETWORK GENERATE is independent and as a central institution and contact and cooperation partner for scientists, doctors, patients and their organizations.
The primary objective of the network is to improve the diagnosis, therapy and care of patients with autoimmune encephalitis. GENERATE assumes that there are numerous additional, previously unknown target antigens in patients with autoimmune encephalitis. Moreover, the underlying pathophysiology and pathogenesis of the related disorders have only been rudimentarily elucidated thus far. To further improve early detection and evolve the most specific treatment modalities, the GENERATE Network fosters tight cross-disciplinary collaboration and utilizes the numerous overlaps within the groups working on basic research and clinical practice at the participating centers. The goal is to ensure that new evidence relating to diagnostic and therapeutic methods can be optimally fast-tracked.
The GENERATE network is a research-initiated, clinical and scientific network. Scientific projects are proposed by GENERATE members and communicated to the participating centres via the GENERATE mailing list after an audit by the Board of Directors. The centres decide on your contribution after the opt-in/opt-out (see chart below).
Structured processes for project proposals:

In the following, you will find brief descriptions of the research projects pursued by the individual clinical and basic research working groups.
Long-term course of NMDA, LGI1, CASPR2 and GAD antibodies of associated autoimmune encephalitis and treatment response to rituximab
Long-term data on prognosis and outcome of autoantibody-associated encephalitis under immunotherapy with rituximab are still lacking. It is not known how many patients in Germany are treated with it and what the response to this therapy is. Objective: An analysis of the long-term course of patients with NMDA, LGI1/ CASPR2 and GAD anti-body-associated limbic encephalitis will be carried out under rituximab therapy and compared to other therapies. Methodology: Retrospective analysis of patient data collected in GENERATE with an observation time of at least 24 months.
Dr. Franziska Thaler (LMU München) Dr. Luise Röpke (Uni Jena), Dr. Christine Strippel (UKM Münster), PD Dr. Nico Melzer (UKM Münster), Prof. Dr. Tania Kümpfel (LMU München), Prof. Dr. Klaus-Peter Wandinger (UKSH Lübeck), PD Dr. Frank Leypoldt (UKSH Kiel)
Visual working memory in anti-NMDA receptor encephalitis
Recurrent glutamaterge neurotransmission at the NMDA receptor plays a crucial role in maintaining memory content. Using functional MRI, we want to under-study memory-related activation during a visual work memory task in patients with anti-NMDAR encephalitis. Patients with anti-NMDAR encephalitis are included up to 6 months after discharge from the acute hospital.
Prof. Dr. med. Carsten Finke (Charité Berlin NeuroCure)
Overlap MS Case Series - NMDA
Clinical project on the possible autoimmune genesis of TLE-AE or seronegative LE. Overlap between clinical relapsing or progressive multiple sclerosis with typical MR lesions and clinical temporal lobe epilepsy with MR changes typical of LE. Inclusion criteria: Multiple sclerosis according to the revised criteria of 2017 as well as seronegative limbic encephalitis according to Graus et al. 2016.
PD Dr. Nico Melzer (UKM Münster)
Genetic predisposition of (auto-)immune encephalitis with GAD65 autoantibodies
Association of HLA characteristics and other genomic markers with autoimmune encephalitis with GAD65 autoantibodies (limbic encephalitis, cerebellitis, stiff person syndrome) compared to type 1 diabetes mellitus and other encephalitis subgroups (anti-NMDA-R encephalitis, anti-LGI1 encephalitis). Patients with autoimmune encephalitis with GAD65 autoantibodies. Case number analysis n=100 anti-GAD65 encephalitis; comparison group anti-NMDA-R encephalitis (n=166, already sequenced) and anti-LGI1 encephalitis (n=75, already sequenced); Control group >1300 healthy controls (already sequenced).
Dr. Christine Strippel (UKM Münster), PD Dr. Frank Leypoldt (UKSH Kiel), Prof. Dr. Monika Stoll (UKM Münster), PD Dr. Nico Melzer (UKM Münster)
Autoimmune encephalitis with GAD antibodies: frequency, clinical symptoms and therapy response - a GENERATE study (GAD-GENERATE)
The aim of the project is the systematic characterization of all patients with GAD-AIE included in the GENERATE network by retrospectively collecting the clinical data of patients with GAD-AIE from the GENERATE database.
Dr. Marie Madlener (UKM Münster), PD Dr. Nico Melzer (UKM Münster), PD Dr. Michael Malter (UK Köln)
Effectiveness of immunotherapy in Iglon5 syndrome: retrospective data analysis
Evaluation of the effectiveness of immunotherapies in Iglon5 syndrome depending on the time of start of therapy and the therapy used or clinical symptoms in the foreground.
Dr. Thomas Grüter, PD Dr. Ilja Ayzenberg (St. Josef Hospital Bochum)
Anti-NMDA receptor associated encephalitis and multiple sclerosis
Under the proposed project, all GENERATE centres will be contacted with a request for notification within 4 weeks (demand for 2 and 3 weeks) as to whether patients with MS or MS-like diseases are present in their NMDAR-AE collective. At the same time, it will be asked whether the basal demographic data and the data necessary to verify the diagnosis according to the grey criteria can be evaluated by all NMDAR-AE patients of the respective centre. This allows NMDAR-AE patients from the centers from which we receive a response, positive or negative, to form the overall cohort with which patients with MS + NMDAR-AE can be compared.
PD Dr. med. Jan Lewerenz (UK) Ulm
Liquid Biopsy in immunotherapy-naive, "tumor-negative" paraneoplastic neurological disease with onco-neural autoantibodies
Can individual circulating tumor cells ("liquid biopsy") be reliably isolated in "tumor-negative" patients with newly diagnosed PND with clear detection of onco-neural autoantibodies? After the isolation of circulating tumor cells, can it be diagnosed by molecular analysis the type of the underlying tumor? On the basis of the findings, can a personalised oncological therapy based on the tumor cell detection be justified and does this possibly lead to a remission of pND? Inclusion criteria: Immunotherapy-naive, "tumor-negative" patients with PND with clear detection of onco-neural autoantibodies.
PD Dr. Dr. Oliver M. Grauer, Dr. Christine Strippel (UKM Münster), Prof. Dr. Christoph Klein (UK Regensburg), PD Dr. Nico Melzer (UKM Münster)
Epileptic seizures and prodromal syndromes in antibody-associated autoimmune encephalitis: frequency and semiology
Although epileptic seizures are a common symptom of autoimmune encephalitis (AIE) (Graus et al., 2016), there are few systematic studies on the specific frequency and semiology of epileptic seizures in the individual subforms of AIE. Case reports show that different forms of epileptic seizures can occur in AIE. In anti-LGI1 encephalitis, faziobrachial dystone seizures (FBDS) as well as isolated temporal lobe attacks may occur. Also within the group of temporal lobe attacks, different seizure manifestations are observed. For example, seizures with a pilomotor symptomatic ("training of a goosebumps") have been described repeatedly, whereby it is discussed whether this type of seizure is a specific clinical characteristic of special forms of aAIE (Rocamora et al., 2014).
Dr. Albrecht Kunze, Dr. Tillmann Fritzel, Prof. Dr. Christian Geis (UK Jena), PD Dr. Michael Malter (UK Köln)
CNS Infections and Autoimmune Cephalitis (EBV)
From the patient collective with autoimmune encephaloids of the GENERATE network, antibodies against the Epstein-Barr virus are to be measured in cerebrospinal fluid and serum samples and intrathecal antibody production is determined. In addition, it will be analyzed whether the outcome differs between ASI positive and ASI negative patients 1 year after the onset of the disease. VCA and EBNA IgG are measured in cerebrospinal fluid and serum using ELISA (Serion ELISA). This part of the project is in cooperation with Jan Lewerenz (Ulm) and Peter Lange (Göttingen). In addition to determination of the ASI, the titter is correlated with the course and comparison with matched control samples from control samples from the Biobank in Hanover and Ulm.
PD Dr. med. Kurt-Wolfram Sühs, Dr. Philipp Schwenkenbecher (MHH Hannover)
Identifying the first events in autoimmune ence-phalitis: Plasma cell trafficking and B-cell-receptor repertoire in autoimmune encephalitis
The aim of the study is the recombinant production of monoclonal human autoantibodies from the blood and cerebrospinal fluid of patients with confirmed autoimmune encephalitis. By comparing clonal expansion, antibody isotypes, distribution of antibody families in the cerebrospinal fluid and peripheral compartment, and by describing the antibody characteristics, it is intended to be possible to understand the development and development of the encephalitis-specific autoantibody repertoire. The study requires cryopreservation and asservation of cerebrospinal fluid cells and PBMCs, the samples are sent to Berlin or Düsseldorf and processed there. It includes male or female patients of all ages with the clinical syndrome of autoimmune encephalitis and the detection of an autoantibody in the cerebrospinal fluid and/or serum against an established autoantigen (e.g. NMDA, GABAa, GABAb, AMPA, mGluR5 receptor, LGI1, Caspr2).
PD Dr. med. Harald Prüss (Charité Berlin), Prof. Dr. med. Norbert Goebels (UK Düsseldorf)
Studying mechanisms of neural network and be-havioural dysfunction in autoimmune encephalitis
The aim of the project is to identify pathological network mechanisms in the hippocampus caused by antibody against the GluN1 subunit of the NMDA receptor. For this purpose, we use eta-faded passive-transfer mouse models and various electrophysiological examination methods in acute hippocampus brain cutting preparations. For the studies, both antibodies from patient liquor and recombinant antibodies against the GluN1 subunit are used. For these and other projects, we are looking for cerebrospinal fluid samples from patients with NMDA receptor, LGI1, AMPA receptor and GABA B receptor antibodies in the GENERATE network.
Prof. Dr. Christian Geis (UK Jena)
Applying advanced longitudinal structural MRI analyses in autoimmune encephalitis
The aim of this subproject is to analyse the frequency and properties of the brain lesion, the atrophy of the brain and the lesion characteristics of overlapping syndromes in their longitudinal development as biomarkers in patients with AE using available clinical data sets and advanced imaging methods with ultra-high-field MRI. This will help guide treatment decisions, improve counseling and understand the underlying mechanisms of lesion formation and brain atrophy in patients with AE. Inclusion criteria: Routine MRIs from GENERATE centers of patients with NMDARE and LGIE + patients with overlap syndrome: clinical description + pre-MRIs + invitation of the pat. to Berlin for the 7T-MRI .
Prof. Dr. med. Carsten Finke (Charité Berlin NeuroCure), Dr. med. Michael Scheel (Charité Berlin), Prof. Dr. med. Thorsten Bartsch (UKSH Kiel)
Use of intravenous immunoglobulins in patients with antibody-associated autoimmune encephalitis
In what proportion of patients associated with autoantibody encephalitis is treatment with IVIG in the acute phase? To what extent does the outcome of IVIG administration correlate in the acute phase? Is there a link to time, pre-therapy, dosage and frequency of IVIG gifts? Patients with autoimmune encephalitis and detection of appropriate antibodies (NMDA, LGI1, CASPR2, AMPA, GABAb, GAD) in the cerebrospinal fluid and/or serum are included. observation period of patients at least 12 months after the onset of the disease.
PD Dr. med. Kurt-Wolfram Sühs, Dr. med. Dominica Ratuszny, Prof. Dr. med. Martin Stangel (MHH Hannover)
Review of the diagnostic criteria proposed by Graus et al. to the patient of the GENERATE cohort (KritValid)
Based on characteristics of one's own patients, literature and expert opinions, Graus et al. (The Lancet 02/2016) have developed criteria for the diagnosis of autoimmune encephalitis (AE). A distinction is made between "possible autoimmune encephalitis" (entry criteria) and "definite autoimmune encephalitis" (confirmation criteria). By choosing narrow entry criteria, which in the second step lead to the "confirmation" of an AE, in particular by auto-ak detection, the disease of some patients could not be classified as autoimmune encephalitis and these patients could only be treated with a time delay or not at all adequate therapy. The inclusion criteria of the GENERATE database are broader, including patients whose disease is considered autoimmune because the symptoms have improved under immunotherapy. - Verification of the diagnostic criteria of Graus et al. by comparing the patients of the GENERATE database who meet the entry or confirmation criteria of Graus et al. with the patients of the database who only meet the broader inclusion criteria of the database. Identification of possible weaknesses of the criteria of Graus et al..
Prof. Dr. med. Florian Then Berg (UK Leipzig)
Elucidating genetic predisposition in autoimmune encephalitis
In 2017/18, the GENERATE network conducted and published the first genome-wide association study (GWAS) of antibody-mediated encephalitis of subtypes NMDA and Lgi1 (Müller et al. AnnNeurol (2018) 83:863:69). In the very small sample for the subtype Lgi1, in addition to a very strong HLA association, other hopeful signals were found. A weak association with an HLA haplotype has been detected for the NMDA subtype. Now we will carry out a second, much larger, genome-wide association study within the framework of BMBF funding. As with the last publication, we are mentioned by name in a supplement to all physicians who contribute to the study as authors or as members of GENERATE. All patients who have consented to GENERATE and who either have EDTA blood/DNA or a saliva sample are included.
Prof. Dr. med. Gregor Kuhlenbäumer, PD Dr. Frank Leypoldt (UKSH Kiel)
Treating AE: A multicenter randomized, controlled, doubleblinded trial to evaluate efficacy and safety of bortezomib in patients with severe autoimmune encephalitis in GENERATE (BOOST-GENERATE)
GENERATE-BOOST is a multicenter, randomized, controlled and double-blind study to verify the efficacy and safety of bortezomib in patients with severe autoimmune encephalitis. A total of 50 patients will be included over a period of about two years. Randomization is done 1:1 according to computer-based algorithm, stratified by test center. The aim of the study is to examine whether the treatment of patients with autoimmune encephalitis by means of bortezomib leads to an improvement in disease symptoms and a reduction of pathogenic antibody titres. The duration of the study is estimated to be that a maximum of 3 cycles of bortezomib can be administered (9 weeks) and the total duration of the study duration for each patient is 17 weeks. The duration of the study is chosen with a total of 17 weeks and 3 cycles of bortezomib for a sufficiently long period that a clinical improvement of the severely affected patients should be recorded.
Prof. Dr. Christian Geis, Prof. Dr. med. André Scherag (UK Jena), PD Dr. med. Harald Prüß (Carité Berlin)
Improving and expanding the registry of the German Network for Research on Autoimmune Encephalitis (IMPROVE-GENERATE)
Sub-project of the BMBF-funded CONNECT-GENERATE Consortium (SP1) with the aim of improving the data infrastructure and quality and carrying out interim evaluations from the database. Patients with GENERATE consent are included.
Prof. Dr. med. Klaus-Peter Wandinger (UKSH Lübeck), PD Dr. Frank Leypoldt (UKSH Kiel)
Coordinating and advancing repositories within GENERATE (RE-GENERATE)
Establish a decentralized biobank structure for optiamal biosample management and search within GENERATE for CONNECT-GENERATE but also other GENERATE projects. In addition to the IT infrastructure, the SOP of biosample production will also be standardized. Another subporject is the identification of biosamples for external quality controls to be offered to the laboratories of the network. For all patients in GENERATE can be stored in RE-GENERATE samples.
PD Dr. med. Jan Lewerenz (UK Ulm), Prof. Dr. Tania Kümpfel (LMU München), Prof. Dr. Klaus-Peter Wandinger (UKSH Lübeck)