How does this pathological process appear to unfold?
The majority of patients with autoimmune encephalitis test positive for autoantibodies against nerve cell components. These antibodies are typically found in the cerebrospinal fluid (CSF) and blood serum and can be detected using modern assay methods (see Diagnostics). Triggered by the humoral immune response, autoantibodies are produced by plasma cells and B lymphocytes. T lymphocytes, on the other hand, are meaningful in some pathological processes where they sometimes play a role as potential effector cells in certain subtypes of this disease complex or by interacting with the B lymphocytes to cause a specific immune response to neural antigens.
Depending on the localization of the antigens targeted by the autoantibodies, the pathological spectrum is differentiated into two fundamental groups (see also Figure 1):
- Autoimmune encephalitides triggering a specific immune response to membrane-based neuronal antigens. These are predominantly localized around neuronal synapses, which are critical for transmitting nerve impulses in the central nervous system and contain mainly ionotropic and metabotropic neurotransmitter receptors and neuronal adhesion molecules. These are the antibodies that play a pathophysiologically relevant role in the disease process, causing a characteristic symptomatology reflective of the disrupted function of the target antigens (Dalmau 2017). Studies with NMDA receptor antibodies have, for example, proven that the antibodies lead to a reduction in the surface expression of these receptors. This directly affects the signal transmission of NMDA receptors as well as impairing downstream processes, like neuronal plasticity, which is the cause of the typical pathological symptoms (Planaguma 2016, Hughes 2010). Accordingly, patients with this disease also show a reduction in NMDA receptors in the brain. This group of autoimmune encephalitides will almost always respond well to immunotherapy. Here, the objective of therapy is to reduce the pathogenic autoantibodies and deplete or influence the antibody-producing immune cells (see Therapy).
- Encephalitides triggering a specific immune response to intracellular neuronal antigens. These are localized either in the cytoplasm or in the nucleus. Oftentimes, these syndromes occur concomitantly with cancer, which is why they are termed paraneoplastic diseases. The specific antibodies in these diseases—called onconeural antibodies—generally do not have any pathophysiological relevance. What does appear relevant in these diseases are the cellular damage mechanisms mediated by T lymphocytes, where cytotoxic processes destroy neurons and often cause irreversible damage. Accordingly, the response to immunotherapy in patients suffering from this group of diseases is comparatively limited, urgently mandating rapid treatment of a potentially underlying tumor in combination with immunotherapy (see Therapy).
Moreover, there are special subtypes of autoimmune encephalitides with antibodies against proteins in presynaptic nerve endings, primary localized intracellularly (e.g. GAD65, amphiphysin) with or without tumor involvement, but associated with different syndromes (e.g. epilepsy, ataxia or stiff person syndrome). The underlying pathophysiology of the cellular and humoral autoimmunity—yet continuingly unclear—appears to play a role here, too.

Figure 1
Immunohistochemical differentiation between autoantibodies which target neuronal surface proteins (here NMDA receptor antibodies, left, A, C, E) or intracellular antigens (here nuclear antigen, right, B, D, F). (Tissue sections: Hippocampus from rat brain A-D; living primary neurons E and F.) Antibodies against surface receptors stain the neuropil in the hippocampus and also bind to living neurons, whereas antibodies to nuclear antigens do not interact with living neurons (Dalmau, Geis, Graus, Physiol Rev., 2017)
Alongside the seropositive autoimmune encephalitides, there is a spectrum of clinical diseases in which an underlying autoimmune inflammation of the grey matter in the central nervous system appears plausible, without specific autoantibodies or T lymphocytes previously produced by B lymphocytes being detectable. In the coming years, intensive research will provide proof of additional target antigens.
In contrast to the typical paraneoplastic diseases with onconeural antibodies, the pathogenesis of subtypes of autoimmune encephalitis with surface antibodies is still not understood completely. Depending on the subtype and target antigen, the association with cancer may be more or less pronounced. For example, this congruence can range from essentially no association with cancer in patients with LGI1 antibody encephalitis up to a very frequent association with lung cancer in patients with GABA-B receptor encephalitis. Ovarian teratomas possibly containing ectopic neuronal tissue can be present in patients with anti-NMDAR encephalitis. In these patients and cases with concomitant tumors, immune tolerance might be abolished. Immune cells then presented neuronal antigens or cross-reacting antigens (referred to as molecular mimicry), thereby inducing a specific antineuronal immune response. Furthermore, certain viral diseases can trigger a secondary immune response by a similar mechanism after damage to cerebral tissue, where neuronal antigens become the target, e.g. the NMDA receptor (Armangue 2018) (see also Figure 2).
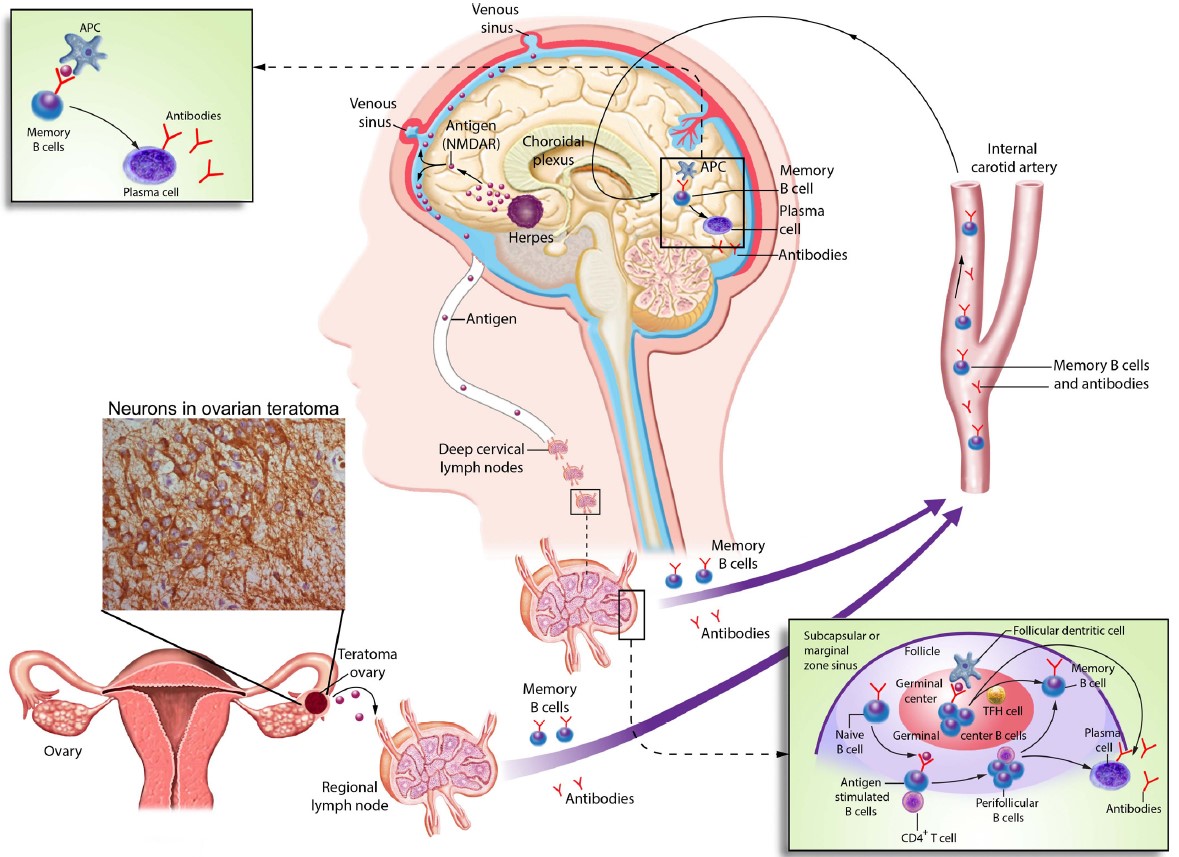
Figure 2
Possible triggers of autoimmune encephalitis, illustrated here using the example of anti-NMDAR encephalitis. Ectopic neuronal tissue in a tumor (here ovarian teratoma) or destroyed cerebral tissue, e.g. secondary to herpes encephalitis, can induce a peripheral immune response, e.g. in the lymph nodes. In interaction with MHC class II molecules and CD4+ T cells, antigen-presenting cells can activate B lymphocytes. Thereby, memory B cells and, ultimately, antibody-producing plasma cells can form. In addition to activated B cells, plasma cells can reach the central nervous system and trigger a central immune response accompanied by local antibody production (Dalmau, Geis, Graus, Physiol Rev., 2017).
References
- Dalmau, J., Geis, C., and Graus, F. (2017) Autoantibodies to Synaptic Receptors and Neuronal Cell Surface Proteins in Autoimmune Diseases of the Central Nervous System. Physiol Rev 97, 839-887
- Planaguma, J., Haselmann, H., Mannara, F., Petit-Pedrol, M., Grunewald, B., Aguilar, E., Ropke, L., Martin-Garcia, E., Titulaer, M. J., Jercog, P., Graus, F., Maldonado, R., Geis, C., and Dalmau, J. (2016) Ephrin-B2 prevents N-methyl-D-aspartate receptor antibody effects on memory and neuroplasticity. Ann Neurol 80, 388-400
- Hughes, E. G., Peng, X., Gleichman, A. J., Lai, M., Zhou, L., Tsou, R., Parsons, T. D., Lynch, D. R., Dalmau, J., and Balice-Gordon, R. J. (2010) Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 30, 5866-5875
- Armangue, T., Spatola, M., Vlagea, A., Mattozzi, S., Carceles-Cordon, M., Martinez-Heras, E., Llufriu, S., Muchart, J., Erro, M. E., Abraira, L., Moris, G., Monros-Gimenez, L., Corral-Corral, I., Montejo, C., Toledo, M., Bataller, L., Secondi, G., Arino, H., Martinez-Hernandez, E., Juan, M., Marcos, M. A., Alsina, L., Saiz, A., Rosenfeld, M. R., Graus, F., Dalmau, J., and Spanish Herpes Simplex Encephalitis Study, G. (2018) Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol 17(9), 760-772



